BOC Sciences offers synthetic polyethylene glycol modification and bioconjugation services by site specific ligation of PEG to peptides, proteins, oligonucleotides, antibodies, lectins, enzymes, toxins, drugs or other small molecules. Our knowledge, skills and capabilities will put your project on the right track and provide an enhanced value proposition.
Polyethylene glycol modification, or PEGylation, is the chemical coupling of activated PEG to proteins, peptides or other molecules by covalent bonds. Since Davies first modified bovine serum albumin with PEG in 1977, PEG modification technology has been widely used in the chemical modification of a variety of proteins and peptides, and a number of PEG modification drugs have been put on the market or in clinical studies. PEG modification has the characteristics of prolonged half-life, decreased or disappeared immunogenicity, reduced toxicity and side effects, and enhanced physical, chemical and biological stability. The drug molecules modified by polyethylene glycol have better pharmacological and pharmacological properties than unmodified drugs.
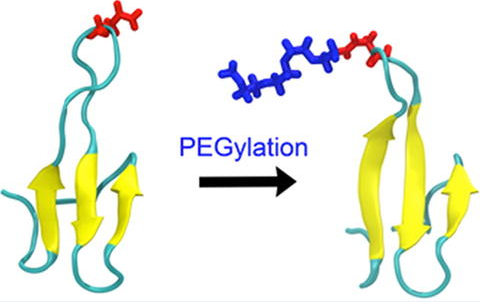 Figure 1. Structural change of molecule by PEGylation1
Figure 1. Structural change of molecule by PEGylation1
By conjugation of PEG to protein, some proteins' properties, for example hydrophobicity, change significantly. The decrease of hydrophobicity benefits protein performance in some applications, especially in drug delivery where PEG conjugation opens up the possibility of accessing desirable properties such as an increased stability due to conjugation, solubility, bioavailability, half-life, decreased immunogenicity, and many more.
The effects of PEGylation on the pharmacokinetics of peptides include avoiding (RES) clearance in reticular endothelial cells, reducing immunogenicity, reducing the loss caused by enzymatic protein hydrolysis and renal filtration, and potentially beneficial changes in biological distribution. These effects can greatly prolong the half-life of peptides in vivo and potentially improve bioavailability, but will not adversely affect the binding and activity of peptide ligands.
The polyethylene glycol coating on the surface of nanoparticles can prevent surface aggregation, conditioning and phagocytosis, and prolong the body cycle time. There are currently more than 35 US FDA-approved NPs often incorporating polyethylene glycol (PEG), with a larger number in preclinical studies for both imaging and therapy.
PEGylation, as a combination of poly(ethylene glycol) (PEG), has been used to improve the pharmacokinetic characteristics of oligonucleotide drugs for nearly 30 years. PEGylation is a method to increase the solubility of oligonucleotides and prevent their rapid elimination, thus increasing tissue distribution.
PEGylation can overcome the short half-life of antibody fragments in blood circulation, reduce the cost of antibody fragments, and make them useful for clinical treatment. Compared with the prototype drug, PEG conjugated antibody has reduced toxicity, and other chemical and biological properties have not changed.
The early progress of PEGlytion mainly focused on the N-terminal amino group of lysine. Since then, polyethylene glycol-toluene ester has also been developed for protein coupling through alkylation reactions, but all of these reactions produce non-specific, multiple coupling products.
The polyethylene glycolization of carbohydrates shows some improvements in properties, such as the bioavailability of drugs, especially enzyme inhibitors, or the production of polymers with drug encapsulation properties.
We provide PEGylation services that improve molecular stability, solubility, and performance with controlled and reproducible attachment methods.
Submit your inquiry to request a custom solution.
References
If you have any questions or encounter issues on this page, please don't hesitate to reach out. Our support team is ready to assist you.