BOC Sciences provides synthesis of different types of chiral ligands and related services. The synthesized chiral ligands can be used for the asymmetric synthesis of metal complex catalysts and other applications, fully satisfying the experimental needs of customers.
A logical extension about the application of chiral ligands in stereoselective catalysis has been developed. In general, ligands design in asymmetric catalysis is guided by several simple concepts and principles. For instance, the design of chiral ligands is frequently based on C2-symmetry to reduce the number of diastereomeric intermediates and transition states which play a role in the catalytic cycle. This approach has been vindicated by the successful development of several large families of (“privileged”) chiral ligands, and these chiral ligands nowadays belong to the basic “tool kit” of asymmetric catalysis, such as chiral diphosphines, salen derivatives and bisoxazolines. These privileged families of ligands possess characteristic properties that lead to the induction of high stereoselectivities in their catalytic reactions. As a result, the syntheses of chiral ligands have evolved into a very dynamic, rapidly growing area of research, attracting an increasing number of chemists from various disciplines.

With a significant number of highly selective chiral catalysts based on chiral NHCs, several general trends in the design of new NHC-containing molecular catalysts for stereoselective transformations in organic synthesis have emerged. Six large families of chiral N-heterocyclic carbine ligands have thus recently emerged: NHCs with N-substituents containing centres of chirality; NHC ligands containing chiral elements within the N-heterocycle; NHC ligands containing an element of axial chirality; carbenes containing an element of planar chirality; carbenes joined by a chiral trans-cyclohexanediamine ligand backbone; carbenes incorporating oxazoline units. NHC ligands with different groups can be coordinated with metals such as rhodium, iridium, palladium, ruthenium, etc., and then used in asymmetric catalytic hydrogenation reactions and asymmetric coupling reactions. We can synthesize NHC ligands with various substituents.
 Fig 2. Some methods to synthesize NHC
Fig 2. Some methods to synthesize NHC
Oxazoline is a five-membered heterocyclic compound containing N and O atoms. N atom is not only an electron-donating atom, but also a good coordination group, which can coordinate with various metal ions such as zinc, rhodium, palladium, nickel, and copper. Chiral oxazoline ligands are an important class of ligands, which are widely used in asymmetric catalytic reactions. They have the advantages of diverse structures, easy access to chiral pool, and good asymmetry inducibility. We can provide the synthesis services of different kinds of oxazoline ligands such as Box, PyBox, Phox.
 Fig 3. Examples of chiral oxazoline ligands
Fig 3. Examples of chiral oxazoline ligands
Chiral phosphine ligands have greatly promoted the development of basic research and industrial application technology research. It is very important to design and synthesize chiral phosphine ligands with different structural matrix and different substituents and apply them to various asymmetric catalytic reactions. Chiral phosphine ligands can be classified into chiral monophosphine ligands, chiral bisphosphine ligands and chiral polyphosphine ligands according to the number of phosphorus atoms, and their chirality can be reflected on the phosphine atom, on a certain atom of the side chain or on both chain, or on the entire ligand molecule. Various synthetic strategies in phosphine chemistry have been applied to the preparation of chiral phosphine ligands, and the synthetic routes have been diversified and simplified.
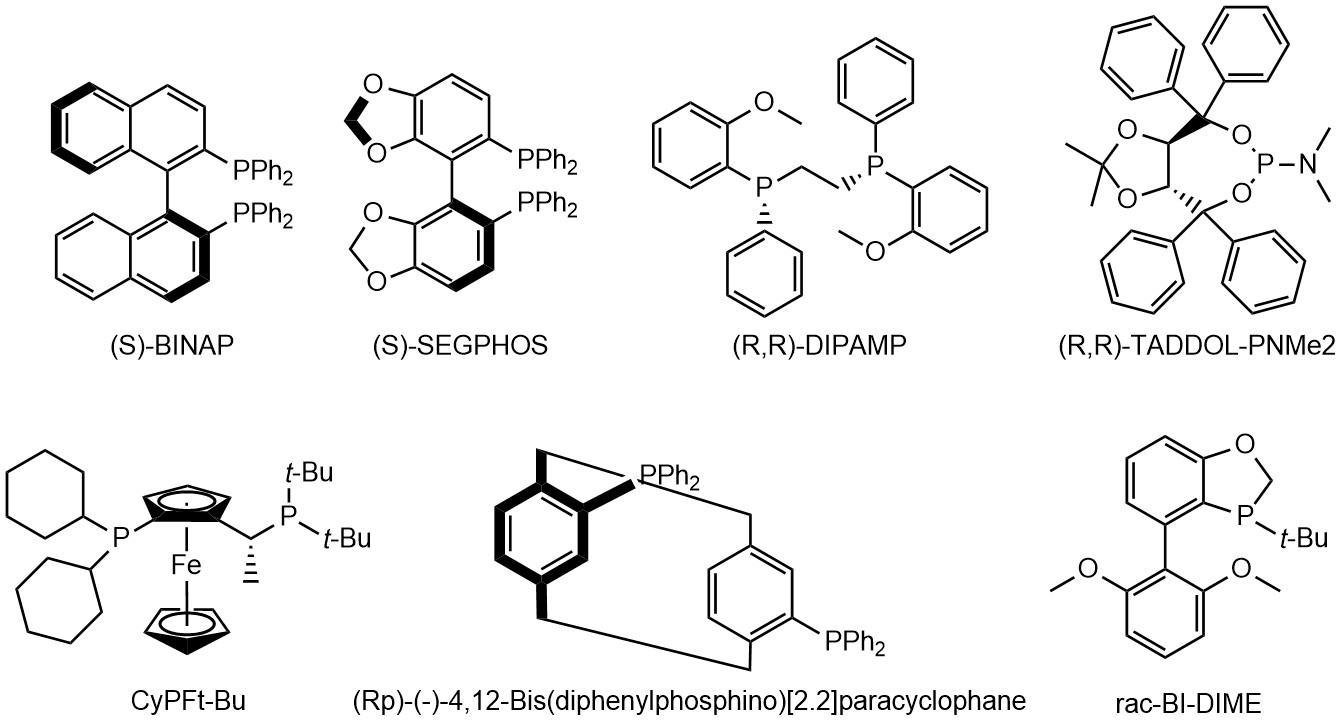 Fig 4. Examples of chiral phosphine ligands
Fig 4. Examples of chiral phosphine ligands
BOC Sciences has extremely rich experience in the synthesis of chiral ligands. We can provide a great deal of chiral ligands and their applications. We have a large number of instruments, as well as many experienced and professional experts. Our company has developed into a first-class modern enterprise with professional knowledge and excellent technical staff. Welcome to get in touch with us, we will provide friendly and professional service for you. Simultaneously, we will continuously improve our professional knowledge and skills to make our company more trustworthy.
We offer chiral ligands with precise structural design to optimize catalytic performance. Our portfolio covers standard and custom ligand solutions for varied synthesis needs.
Submit your inquiry to request a custom solution.
References
If you have any questions or encounter issues on this page, please don't hesitate to reach out. Our support team is ready to assist you.