BOC Sciences provides comprehensive and professional chiral resolution services and synthesis services of chiral resolution agents. The chiral resolution reagents we synthesized have stable properties, high optical purity, easy reaction, and high recovery rate.
Chiral resolution, as an important tool in the production of optically active drugs, is a process for the separation of racemic compounds into their enantiomers in the aspect of stereochemistry. Particularly when the methods of stereospecific synthesis designed for one of the enantiomers, at present, lead generally to enhanced enantioselectivity, it is not only the development of analytical methods for the chiral separation to control optical purity of a large number of pharmaceuticals/therapeutics, administered today, but also the development of methods for quantitative large-scale separation from their racemic or enantiomeric mixtures. These are the requirements and challenges of chemists, and chiral resolution opens wider perspectives for such developments.
 Figure 1. Methods to obtain enantiomerically pure compounds by means of chiral resolution
Figure 1. Methods to obtain enantiomerically pure compounds by means of chiral resolution
Our team has resolved a wide variety of chiral compounds, including:
These examples illustrate the versatility of our chiral resolution services across diverse chemical classes and highlight our ability to tailor methods to specific client needs.
The separation of racemates into pure enantiomers through crystallization is an important industrial process. The crystallization method has the advantages of simple operation, high product purity, and easy realization of industrial production. Preferential crystallization (seed method) is to add a single enantiomer seed to the supersaturated solution of the racemic mixture to induce the preferentially crystallization of enantiomer and achieve resolution. Spontaneous crystallization means that when the racemate is crystallized, a mixture of enantiomerically pure crystals (the so-called agglomerate) is spontaneously formed, and then both enantiomers precipitate spontaneously in equal amounts in the form of enantiomeric crystals.
 Figure 2. The process of two direct crystallization methods in crystallization resolution methods
Figure 2. The process of two direct crystallization methods in crystallization resolution methods
Chemical resolution is a resolution method that uses a chiral resolution agent to resolve racemates into single optical isomers. Chiral resolving agents can form salt bonds with racemates to obtain diastereomeric salts. According to the difference in solubility, the two diastereomeric salts can be separated by filtration and purified by crystallization, and finally the resolving agent can be removed and a pair of enantiomers can be obtained respectively. If the racemate is a true racemic mixture, it cannot be separated by preferential crystallization, but can be resolved using the diastereomer crystallization.
 Figure 3. The process of diastereomeric crystallization
Figure 3. The process of diastereomeric crystallization
Resolving agents include acidic resolving agents, organic base resolving agents and other resolving agents. Acidic resolving agents are suitable for resolving acidic compounds, such as tartaric acid (TA) and its derivatives. Alkaline resolving agents are suitable for resolving basic compounds, such as cinchonine, cinchonidine, and phenethylamine.
The third method used in the resolution of racemates is kinetic resolution. The success of this method is dependent on the fact that the two enantiomers react at different rates with the chiral entity. The chiral entity should be present in catalytic amounts, and it may be a biocatalyst (enzyme or microorganism) or a chemocatalyst (chiral acid or base, or even a chiral metal complex). Kinetic resolution of racemic compounds is by far the most common transformation catalyzed by lipases.
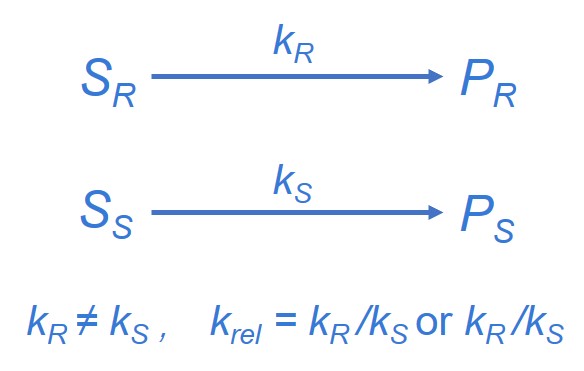 Figure 4. Principle of kinetic resolution
Figure 4. Principle of kinetic resolution
Enzyme-catalyzed kinetic resolution is the stereoselective catalysis of an enantiomer in a substrate by an enzyme, so that one of the enantiomers can produce products faster, and finally achieve chiral resolution. Enzymes commonly used for kinetic resolution include lipase, transaminase, etc. Lipase-catalyzed kinetic resolution of racemates is considered to be one of the most fascinating topics in asymmetric catalysis. Most lipases accept a broad range of non-natural substrates and are thus very versatile for applications in organic synthesis. In many cases, they exhibit good or even excellent stereoselectivity. Lipases have been widely used in three main types of reactions that yield enantiomerically pure compounds. These three types of reactions are kinetic resolutions of racemic carboxylic acids or alcohols, enantioselective group differentiations of meso dicarboxylic acids or meso diols, and enantiotopic group differentiation of prochiral dicarboxylic acid and diol derivatives.
Non-enzymatic kinetic resolution catalysts include chiral organometallic complexes and chiral small molecule organocatalysts. Organometallic complexes are complexes formed by coordination metals and chiral ligands. Organometallic complexes enantioselectively catalyze certain enantiomers to achieve chiral resolution. The coordination metal is generally a transition metal such as titanium, ruthenium, rhodium, and palladium.
BOC Sciences has accumulated abundant experience in chiral resolution, and we can provide comprehensive and professional services for your requirements. Our featured chiral resolution services include resolution by crystallization, resolution by Lipase-mediated organic solvents and so on. There are a large number of instruments and enormous seasoned experts in our company. BOC Sciences' mission is to use professional technologies and knowledge to benefit customers.
BOC Sciences offers end-to-end chiral resolution services designed for research and industrial clients. Our workflow includes:
What is the chiral resolution technique?
Chiral resolution is the process of separating enantiomers—molecules that are mirror images of each other—into their pure optical isomers. Techniques include crystallization with chiral resolving agents, chromatographic separation on chiral stationary phases, and enzymatic resolution.
What is the resolution of a chiral acid?
Resolution of a chiral acid involves separating its enantiomers, often through the formation of diastereomeric salts with chiral bases, followed by crystallization. The resulting pure enantiomers are recovered by reversing the salt formation.
What is the resolution of chiral alcohols?
Chiral alcohols can be resolved using methods such as esterification with chiral acids, enzymatic transesterification, or separation by chiral chromatography, allowing isolation of each optical isomer in high enantiomeric purity.
What does it mean to say that a drug is chiral?
A chiral drug contains one or more stereocenters, resulting in enantiomers that may exhibit different biological activities or interactions. Identifying and isolating the active enantiomer is often critical for research and development.
What chiral resolution capabilities does BOC Sciences provide?
BOC Sciences offers comprehensive chiral resolution services, including chromatographic separation, crystallization, and enzymatic techniques. Our expertise covers chiral acids, alcohols, amines, and other stereochemically complex molecules to support research and development projects.
We provide optimized chiral resolution methods to achieve high-purity enantiomers, combining technical expertise with scalable production capability.
Submit your inquiry to request a custom solution.
Reference
If you have any questions or encounter issues on this page, please don't hesitate to reach out. Our support team is ready to assist you.