As a leading contract research organization, BOC Sciences provides comprehensive API synthesis services, offering high-quality, scalable, and customizable solutions. Leveraging advanced synthetic methodologies and stringent quality control, we ensure efficient production of complex small molecules and intermediates with consistent reliability for pharmaceutical development and manufacturing.

Active Pharmaceutical Ingredient (API) is defined by the U.S. Food & Drug Administration as: any substance or mixture of substances intended to be used as the active ingredient in the manufacture of a drug (medicinal) product. Such substances are intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or function of the body.
API may exist in the form of liquids, powders, crystals, and extracts that are obtained by chemical synthesis, plant extraction, or biotechnology, and are not taken by patients directly. Only when the API is processed into a pharmaceutical preparation can the product be used for therapeutic use. The synthesis of APIs is usually a complicated and multi-step process involving numerous chemical transformations and operations on a range of raw materials with different physical and chemical properties. Specialized expertise is needed to achieve the synthesis of these molecules. BOC Sciences, with seasoned chemists, would provide a comprehensive supply of guaranteed API synthesis service.
The categorization of Active Pharmaceutical Ingredients is a multifaceted endeavor, dependent on their unique chemical architecture and origin. To that end, the triumvirate of API classes is: Synthetic APIs, Biologic APIs, and Natural APIs.
Synthetic APIs: Synthetic APIs, for instance, are purposefully crafted to emulate the intricate design and functionalities of natural compounds, including proteins, enzymes, and hormones. Notably, the pharmaceutical industry widely employs these APIs as they possess a facile manufacturability, longevity, and can be tinkered with to optimize their unique features.
Biologic APIs: Conversely, Biologic APIs are procured from living organisms, namely cells, tissues, or microorganisms, making their molecules markedly intricate and necessitating unique fabrication techniques, like cell culture, fermentation, and purification.
Natural APIs: Lastly, Natural APIs are extracted from organic resources like plants, animals, or minerals. Natural APIs have emerged as potential sources of new drugs, albeit with the notable tradeoff of being less potent and specific than their synthetic or biologic counterparts. Nonetheless, natural APIs exhibit the salient benefit of being more readily obtainable and frequently induce fewer side effects.
There are certain inorganic molecules that can be used as active pharmaceutical ingredients. Taking the aluminium hydroxide (the synthesis route is displayed as below) as an example, which is a typical inorganic API that reacts with excess gastric acid to reduce the acidity in the stomach, it can significantly relieve the symptoms, including ulcer, indigestion, and heartburn.
2NaOH + CO2 = Na2CO3 + H2O
NaAl(OH)4 = Al(OH)3↓+NaOH
Organic synthetic APIs are produced mainly by basic organic chemicals and underwent a series of organic chemical reactions. Clofarabine, a promising DNA polymerase inhibitor currently under investigation, helps the inhibition of DNA repair in leukemic lymphocytes.
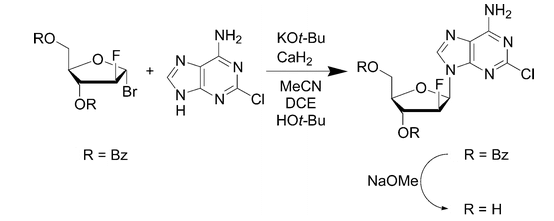 Fig.2 Biosynthesis primed with acetate via the decarboxylation of malonate (a) and benzoate (b).
Fig.2 Biosynthesis primed with acetate via the decarboxylation of malonate (a) and benzoate (b).
Organic biosynthesis is an important strategy for API synthesis. The multienzyme total synthesis of the maritimus enterocin wailupemycin bacteriostatic agents and streptomyces are reported in a single reaction vessel from benzoate and malonate substrates (Fig. 2).
 Fig. 2. Biosynthesis primed with acetate via the decarboxylation of malonate (a) and benzoate (b).
Fig. 2. Biosynthesis primed with acetate via the decarboxylation of malonate (a) and benzoate (b).

Building blocks are important intermediates for many drugs and have major significance in the discovery and synthesis of drugs. Many important substances and some significant effects of natural drugs and synthetic drugs, such as chlorophyll, heme, and nucleic acid, contain a heterocyclic structure.

Owing to chirality is a pivotal factor in the safety and efficacy of many drug products, the production of single enantiomers of drug intermediates has become increasingly important in the pharmaceuticals industry. It has been seen that the increasing awareness of the potential of microorganisms and enzymes derived from the transformation of synthetic chemicals with intermediates.

Impurity profile studies play significant roles in active pharmaceutical ingredient (API) development, which are closely related to the quality, safety, and efficacy of drug products. Tailored impurity synthesis supports structural elucidation, process optimization, and ensures a deeper understanding of product consistency.

Metabolites of pharmaceutical compounds are pivotal to comprehending drug metabolism, potential drug interactions, and human drug metabolizing enzymes. The synthesis and purification of considerable amounts of the metabolites provides reliable conclusions regarding the cardiotoxicity of drug metabolites and then the pharmacological prevention or treatment of the disease side effects.

Reference materials (reference compounds), established for measuring the quality of analysis and are critical for any measurement process characterized by a metrologically valid procedure for one or more specified properties. They provide indispensable benchmarks for method validation, instrument calibration, and long-term quality assurance in pharmaceutical research and development.

Chirality is a fundamental property in pharmaceutical chemistry, as enantiomers of the same compound can exhibit distinct pharmacological activities, safety profiles, and therapeutic effects. BOC Sciences offers advanced chiral synthesis services, enabling the preparation of single enantiomers, diastereomers, and optically pure compounds.
BOC Sciences delivers comprehensive API synthesis services, combining deep expertise in multi-step organic synthesis, chiral chemistry, route optimization, and impurity profiling to produce high-quality, scalable, and fully customizable chemical entities. Our integrated capabilities are supported by a wide array of advanced instrumentation and analytical platforms, ensuring precise synthesis, rigorous structural verification, and consistent purity. In addition to the core tools commonly referenced, we maintain an extended suite of specialized equipment to meet complex and diverse project requirements efficiently and reliably.




BOC Sciences provides efficient synthesis of small molecule APIs, covering route design, process optimization, and scalable production. Our expertise ensures high purity, reproducibility, and consistent yields, supporting both research and larger-scale production needs.
Our team is skilled in producing peptide APIs through both solid-phase and liquid-phase approaches, including fragment coupling and controlled oxidation/folding. This enables the production of high-quality peptides with precise sequences and optimized physicochemical properties for research and development applications.
Our oligonucleotide synthesis services cover phosphoramidite-based methods, modified nucleotides, and efficient purification. We provide high-purity oligonucleotides for diverse applications, ensuring accurate sequence fidelity and reliable performance in downstream processes.
We deliver polymer-drug conjugates, including PEGylation and dendrimer-based constructs, using advanced conjugation chemistries. Our platform ensures precise control over polymer length, drug loading, and molecular uniformity to support research and specialized applications.
BOC Sciences offers ADC synthesis services encompassing linker design, payload conjugation, and drug-antibody ratio control. Our expertise ensures reproducible conjugation efficiency and homogeneous product distribution suitable for experimental and preclinical research purposes.
We provide customized lipid API synthesis, including cationic, ionizable, and PEGylated lipids. Our controlled microfluidic and extrusion technologies ensure consistent particle properties, purity, and scalability for research and formulation applications.
We provide radiolabeled API synthesis services, including 14C, 3H, and 131I, employing precise and controlled labeling strategies. We provide high-specific-activity compounds with rigorous quality control suitable for research applications requiring isotopic tracing.
We provide 13C, 2H, and 15N-labeled API synthesis, offering complete or site-specific incorporation. Our methods guarantee precise isotopic enrichment and maintain structural integrity for advanced research and analytical applications.
We isolate natural products from diverse sources to provide high-quality starting materials for chemical development. Through efficient extraction, purification, and analytical verification, we deliver reproducible, high-purity natural product APIs that support discovery research and early-stage projects.
Natural compounds are transformed into semi-synthetic APIs via selective chemical modification. Our process integrates controlled reactions, purification, and quality verification, ensuring consistent, high-purity products suitable for research and early-stage drug development.
We construct complex polycyclic APIs through multi-step synthesis using advanced strategies like flow chemistry. Our approach ensures efficient, reproducible production of challenging molecular scaffolds, supporting both small-scale research and larger-scale development.
Our expertise in heterocyclic API synthesis allows the creation of diverse molecules with precise functionalization. Techniques such as microreactor and continuous flow chemistry ensure reproducibility, scalability, and high-quality heterocyclic compounds for research and early development.
We synthesize APIs with high precision, purity, and reproducibility, supporting both research and production needs. Leveraging advanced synthetic methodologies, we handle a wide range of complex molecules and intermediates with efficiency and reliability. Our integrated quality control system, including HPLC, LC-MS, and NMR analyses, ensures every batch meets stringent specifications. Scalable and customizable services allow seamless transition from laboratory-scale synthesis to larger production, providing dependable support throughout your drug development and manufacturing projects.
BOC Sciences' priority is to provide the highest quality APIs to our customers. Our experts have a wealth of experience in the synthesis of APIs with various starting materials and intermediates. The products will be provided with integrated data packages including chemical purity (GC/MS, HPLC, LC/MS) and concentration (mass spectrometry and NMR). We are pleased to hear from you and look forward to working with you.

The initial phase of Active Pharmaceutical Ingredient (API) development necessitates the identification of a prospective target molecule that can trigger the desired therapeutic effect, known as Target Identification. This primary stage can be executed using an array of methods, including target-based screening, phenotypic screening, or computer-aided drug design, to name but a few.

Once a target molecule has been pinpointed, the next phase is to increase its efficacy and diminish its toxicity by optimizing its properties, a process referred to as Hit-to-Lead Optimization. Consequently, researchers endeavor to synthesize and assay a sequence of analogs or derivatives of the target molecule to pinpoint the most promising lead compound.

Following the identification of the lead compound, researchers initiate Lead Optimization, whereby the optimization procedure amplifies its pharmacokinetic and pharmacodynamic properties by modifying its chemical structure. Modifications are geared toward enhancing the compound's absorption, distribution, metabolism, and excretion.

Upon the successful completion of Lead Optimization, preclinical development ensues, whereby the optimized lead compound is evaluated in preclinical models to gauge its safety, efficacy, and pharmacokinetics. Animal models, such as rats or mice, are employed to determine the compound's toxicity and effectiveness, and to verify the feasibility of progressing to human studies.

Lastly, once the lead compound is deemed safe and effective, API Manufacturing begins, necessitating several manufacturing steps, including synthesis, purification, and characterization, to yield a high-quality API.
The API synthesis process involves designing an efficient synthetic route, performing stepwise chemical reactions to construct the target molecule, optimizing the synthesis to improve yield and purity, purifying and isolating the API, characterizing its structure and quality through analytical testing, and finally scaling up production while maintaining consistency and reproducibility.
A synthetic API is an active pharmaceutical ingredient produced entirely through chemical synthesis rather than extracted from natural sources. It allows precise control over chemical structure, purity, and yield, making it suitable for large-scale production and consistent pharmaceutical applications.
APIs can be categorized into four main types: small molecule APIs, which are low molecular weight compounds synthesized chemically; biologics, including proteins, peptides, and antibodies; oligonucleotide or nucleoside APIs, comprising short DNA or RNA sequences; and advanced or conjugated APIs, such as antibody-drug conjugates, PEGylated compounds, or lipid-conjugated molecules.
In oncology, an API refers to the active component in a therapeutic formulation responsible for anticancer activity. It can be a small molecule cytotoxic agent, a targeted kinase inhibitor, a monoclonal antibody, or a drug conjugate specifically designed to target cancer cells, determining the therapy's efficacy, selectivity, and pharmacological profile.
References
If you have any questions or encounter issues on this page, please don't hesitate to reach out. Our support team is ready to assist you.