BOC Sciences' pharmaceutical separation and purification team are committed to the separation and identification of various impurities in the process of pharmaceutical research. Our experts are highly recognized by customers with their rich experience and problem-solving skills.
 Figure 1. Main types and sources of drug impurities.
Figure 1. Main types and sources of drug impurities.
Impurity control is one of the core contents of drug quality control, and impurity research runs through the whole process of drug research and development. The isolation and identification of drug impurities are the most direct means for the study of impurities, and the separated impurities can be used for an in-depth study of qualitative, quantitative and safety. BOC Sciences provides comprehensive impurity isolation and identification services.
To achieve quantitative analysis of impurities, we can separate impurities to obtain single components of impurities with good purity. We can add additional tests for impurities, including residual solvent (GC) and water content (Karl Fischer).
| Testing item | Specification | Result |
| Appearance | Brown viscous gel | Conforms |
| Purity (by HPLC) | ≥ 95% | 95.1% |
| 1HNMR, MS, IR | Conforms to structure | Conforms |
| Water content (by KF) | - | 0.6% |
| Ethyl acetate residue (by GC) | - | 3.81% |
| Conclusion | The products compiles with the given specification | |
| Storage | 2 -8 °C. Air sensitive. Keep in a inert atmosphere | |
We can use HPLC, LC-MS, IR, UV-Vis, 1HNMR, 13CNMR, etc. to qualitatively analyze impurities for identification purposes.

Liquid chromatography is widely used in downstream process crossing several industries. The technology becomes standard separation process to remove impurities or undesirable chemicals. Normally, the products are separated by affinity or hydrophobicity interaction with chosen functional columns. However, the state-of-art approach is limited in large biomolecules separation because of the lack of control in pore size. In fact, although size exclusion separation provides higher resolution, the technique is seldom used in the processing step because of the high pressure needed.
In addition to the conventional impurity isolation and identification techniques, we provide an alternative method to remove small molecular impurities below six angstrom with 100 times larger throughput. The technology is developed based on patented materials possess well-defined six angstrom porosity to identify products from impurities. Moreover, the chromatography column is further designed to achieve 100 times better throughput.
The patented materials are self-assembled, highly symmetrical, ultrasmall inorganic cages directed by surfactant micelles, developed by research team at Cornell University.
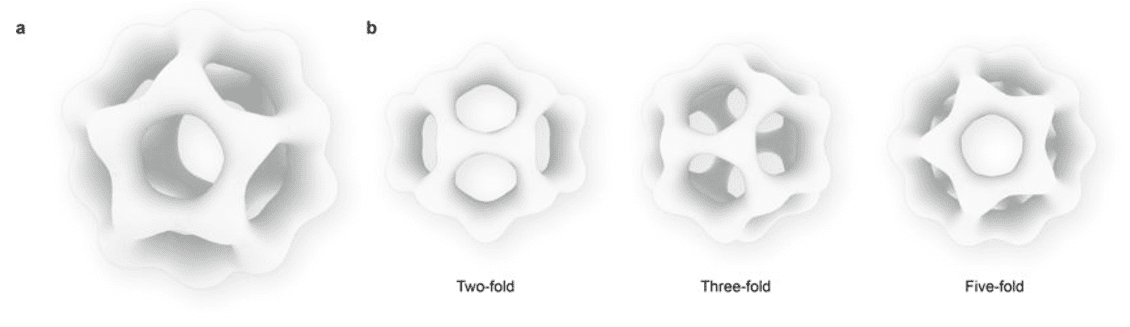 Figure 2. a. Reconstructed dodecahedral silicage. b. Its three most unique projections.
Figure 2. a. Reconstructed dodecahedral silicage. b. Its three most unique projections.
This new technology can be used for removal of precursor or small molecular impurities under 6 angstrom, such as carcinogens, degradation products and other unknown impurities. Our initial proof-of-concept experiment has successfully demonstrated the viability of the technology for the separation of small molecule impurities that have recently led to recalls due to the unacceptable level of NDMA contaminations in the high-blood pressure drug, Ibesartan.
 Figure 3. Illustration of the mechanism of removing NDMA from Irbesartan.
Figure 3. Illustration of the mechanism of removing NDMA from Irbesartan.
Note:
NDMA has attracted wide attention as being highly hepatotoxic and a known carcinogen in lab animals. High levels of exposure can cause reduced function of the kidneys and lungs. To date, NDMA has caused several recalls and warnings of successful drugs on the market, including Valsartan, Losartan, Irbesartan, Ranitidine and Metformin.
The experiment results show that the genotoxic impurity, NDMA in drug products was removed more than 99% (from 2000 ppm to < 1 ppm) by our new technology and the concentration went down below FDA's daily allowance intake. At the same time, the yield of active pharmaceutical ingredient retained more than 90%. The measurements were conducted by following FDA published GC methods. Lower concentration can be achieved by repeating the separation process.
 Figure 4. Efficiency of impurity NDMA removal.
Figure 4. Efficiency of impurity NDMA removal.
Through this technology, we can adjust the porosity of silicages to achieve specific separation purpose, we are now offering services for impurities removal, API purification and product upgrade. Please feel free to contact us if our new technology can help you.
Impurity isolation is a crucial step in API and drug product R&D. By obtaining pure impurities, researchers can investigate reaction mechanisms, degradation pathways, and impurity profiling in greater detail. These insights support process optimization, stability studies, and quality improvement, helping to design more reliable and efficient synthetic routes.
Isolated impurities also serve as essential reference standards in analytical method development and validation. Techniques such as HPLC, LC-MS, IR, UV-Vis, 1H NMR, and 13C NMR rely on well-characterized impurities to achieve accurate qualitative and quantitative results. This ensures reproducibility, enhances analytical confidence, and provides a strong basis for ongoing pharmaceutical research.
Genotoxic or toxic impurities, such as NDMA, are effectively removed and quantified, supporting safety evaluation and toxicological studies. Controlled impurity isolation enables accurate assessment of potential risks and ensures the safety and quality of drug substances throughout development and production.
BOC Sciences isolates and characterizes impurities using advanced purification and analytical techniques.
Submit your inquiry to request a custom solution.
References
If you have any questions or encounter issues on this page, please don't hesitate to reach out. Our support team is ready to assist you.