BOC Sciences is committed to the synthesis and method development of chiral auxiliaries, and can develop and produce more high-quality chiral auxiliaries for our customers.
Chiral auxiliary is a compound or unit that is temporarily added to organic synthesis to control the synthesis of stereochemistry. As the figure below shows, by adding a chiral auxiliary, the prochiral substrate can be transformed into a chiral product. Moreover, the auxiliary can typically be recycled for future use. As an auxiliary for chiral synthesis, it must meet the following conditions: the synthesis procedure must be highly stereoselective; if it is used as raw material, the newly generated chiral center or other chiral elements should be easily separated from chiral auxiliary without racemization; it should has a high recovery rate without reducing the optical purity.
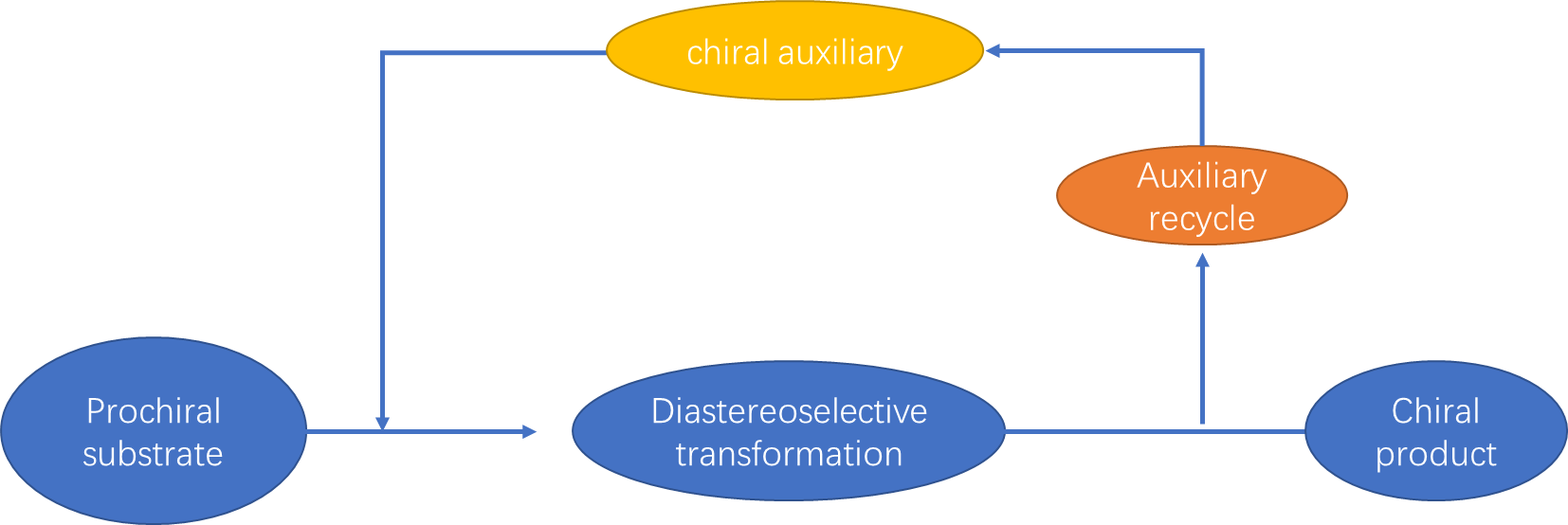 Fig 1. The mechanism model of chiral auxiliary
Fig 1. The mechanism model of chiral auxiliary
Menthol has 3 chiral centers, so it has 8 stereoisomers. Among these isomers, (-)-menthol has the greatest industrial value. (-)-Menthol can be purified from natural mint crude oil or can be obtained synthetically. There are many ways to synthesize (-)-menthol:
 Fig 2 Several methods of synthesizing (-)-menthol
Fig 2 Several methods of synthesizing (-)-menthol
Many (-)-menthol derivatives are also useful chiral aids, such as 8-phenylmenthol. Phenylmenthol can be used to prepare key prostaglandin intermediates in optically pure form without resolution. The back face of the acrylate is blocked by the auxiliary, so that cycloaddition occurs at the front face of the olefin.
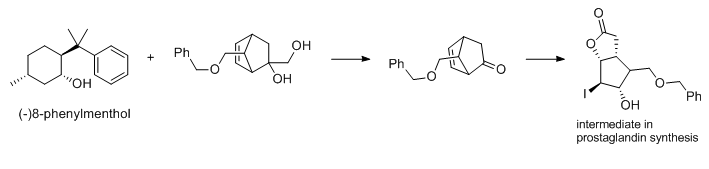 Fig 3 The synthesis of intermediate of prostaglandin using 8-phenylmenthol
Fig 3 The synthesis of intermediate of prostaglandin using 8-phenylmenthol
The oxazolidinone chiral auxiliary reagents can be synthesized using amino acids, amino alcohols or other compounds.
 Fig 4 Synthesis of oxazolidinone compounds by reaction of amino alcohol and dihydrocarbyl carbonate
Fig 4 Synthesis of oxazolidinone compounds by reaction of amino alcohol and dihydrocarbyl carbonate
 Fig 5 Synthesis of Evans reagent using amino acid derivatives as starting materials
Fig 5 Synthesis of Evans reagent using amino acid derivatives as starting materials
Oxazolidinone auxiliaries, popularized by David Evans, have been applied to many stereoselectivity conversions, including the aldol reactions, alkylation reactions, and Diels-Alder reactions. Usually, the acid chloride substrate reacts with the oxazolidinone to form an imide. Substituents at the 4 and 5 positions of the oxazolidinone direct any aldol reaction to the alpha position of the carbonyl of the substrate.
Enantiomerically pure sulfoxides can be efficient chiral controllers, which are cheap and easy to introduce and functionalize. Therefore, they are versatile intermediates in a number of organic reactions. Introduction of the sulfinyl groups mostly occurs by nucleophilic addition of the deprotonated sulfoxides to esters to form β-keto sulfoxides.
(S)-1-amino-2-methoxymethylpyrrolidine (SAMP) and (R)-1-amino-2-methoxymethylpyrrolidine (RAMP) have become commonly used chiral auxiliaries in organic synthesis. The auxiliary SAMP is available starting from (S)-proline, RAMP can be synthesized from (R)-glutamic acid.
 Fig 6 Synthesis of SAMP and RAMP
Fig 6 Synthesis of SAMP and RAMP
Amino alcohol is an important chiral auxiliary to form chelate. Ephedrine derivatives are especially popular in this respect of chiral auxiliary. The asymmetric alkylation of substrates attached to pseudoephedrine is a highly efficient method for the synthesis of optically active carboxylic acids or amino acids.
Camphor molecule has two chiral centers, but due to the immobilization of the bridge ring, there are actually only one pair of optical enantiomers, namely the right-handed (1R) (+) type and the left-handed (1S) (-) type. Its rigid backbone is an attractive structural element of chiral auxiliaries. As a consequence, many camphor-derived auxiliaries with diverse structures have been prepared in only a few steps and successfully employed in a variety of different reactions.
 Fig 7 Camphor and its derivatives
Fig 7 Camphor and its derivatives
The carbohydrate-derived auxiliaries have many stereocenters. They have been successfully employed in numerous organic reactions, such as cycloadditions (Diels–Alder reactions, [2+2] cycloadditions), cyclopropanations, alkylations, and Mannich reactions.
Experts with extensive experience in chiral auxiliaries synthesis in BOC Sciences can help provide pure optical chiral auxiliaries with the advantages mentioned above to meet your specific needs. We also use multiple techniques (NMR, LCMS, GCMS, X-ray) to analyze compounds after the chiral synthesis is completed. If you have any chiral auxiliaries synthesis requirement in mind, please do not hesitate to contact us. We will endeavor to provide highly satisfying products and services!
We design and supply chiral auxiliaries that deliver high stereocontrol, reusability, and compatibility with diverse reaction systems. Our solutions enable efficient asymmetric synthesis with reliable results.
Submit your inquiry to request a custom solution.
References
If you have any questions or encounter issues on this page, please don't hesitate to reach out. Our support team is ready to assist you.